Maedeh Seyedolmohadesin
Hi! I'm a physics PhD student at Northeastern University, where I develop experimental and computational tools to study brain-wide sensory processing underlying sexually-dimorphic behaviors and developmental decision-making. I was responsible for the frontend development of this website. Outside of the lab, I like to try new things; I enjoy playing the piano and painting with watercolors. I also love hiking and backpacking.

Sina Rasouli
I'm an experienced data scientist, programming lover, and PhD student at Northeastern University.
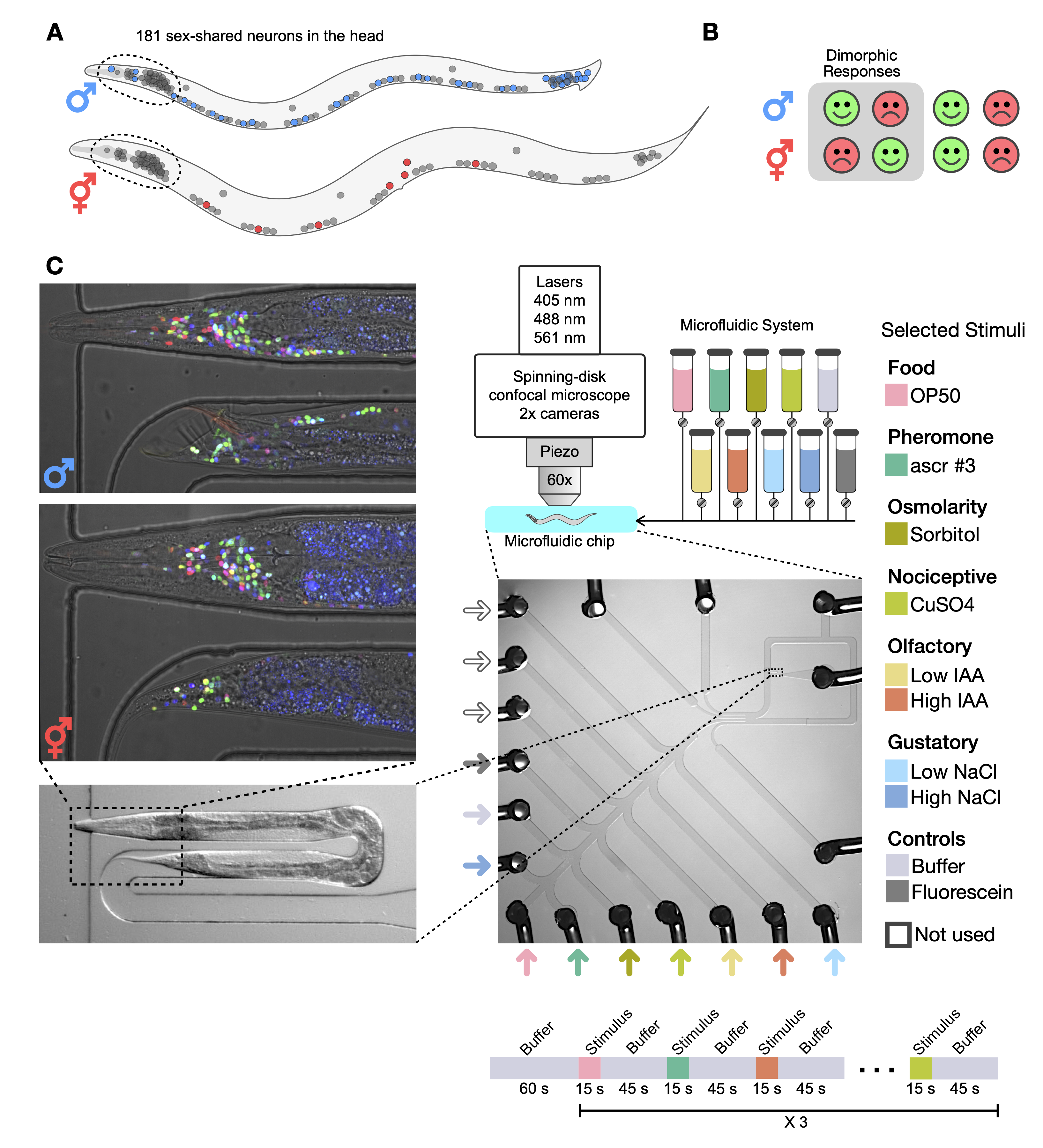
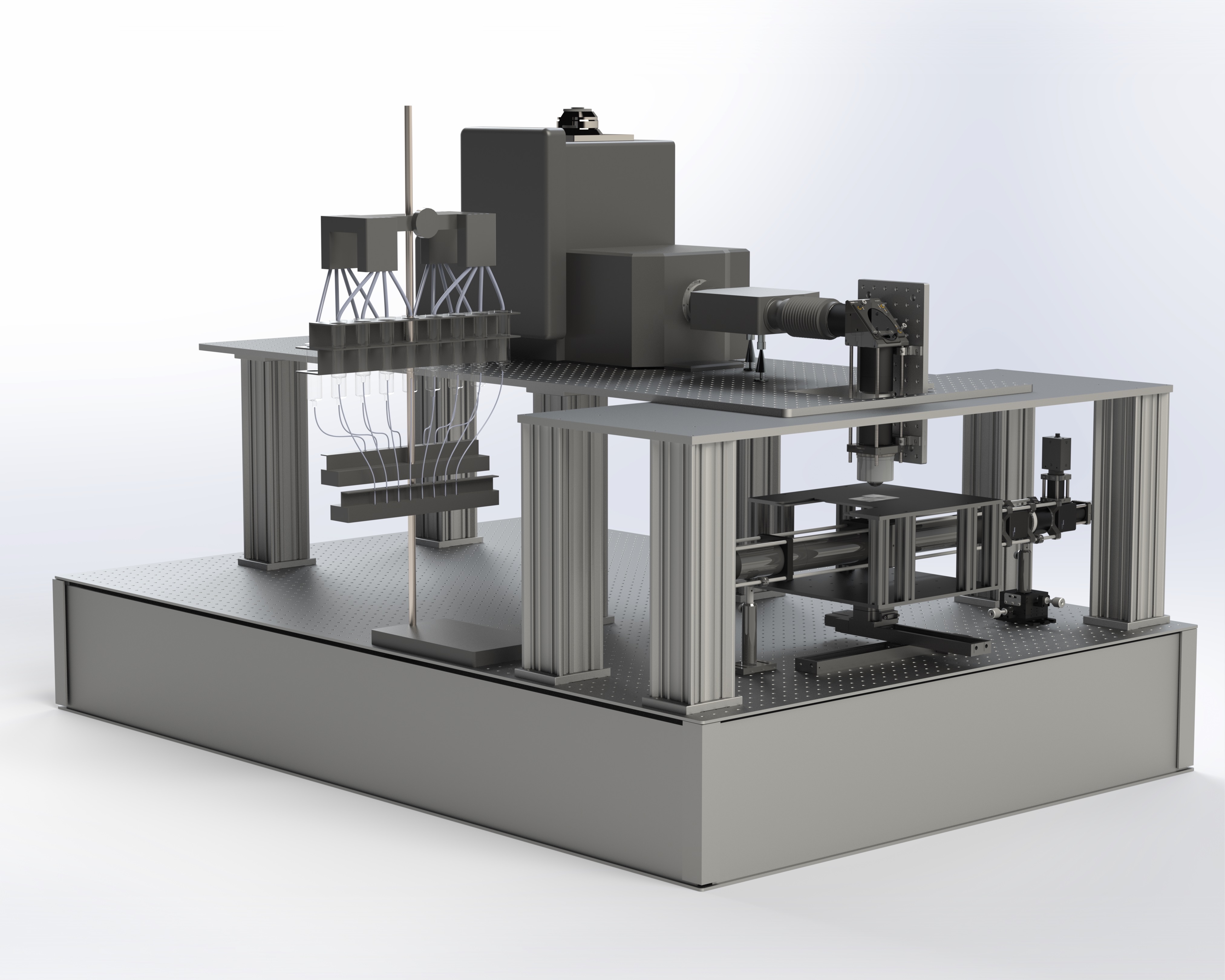
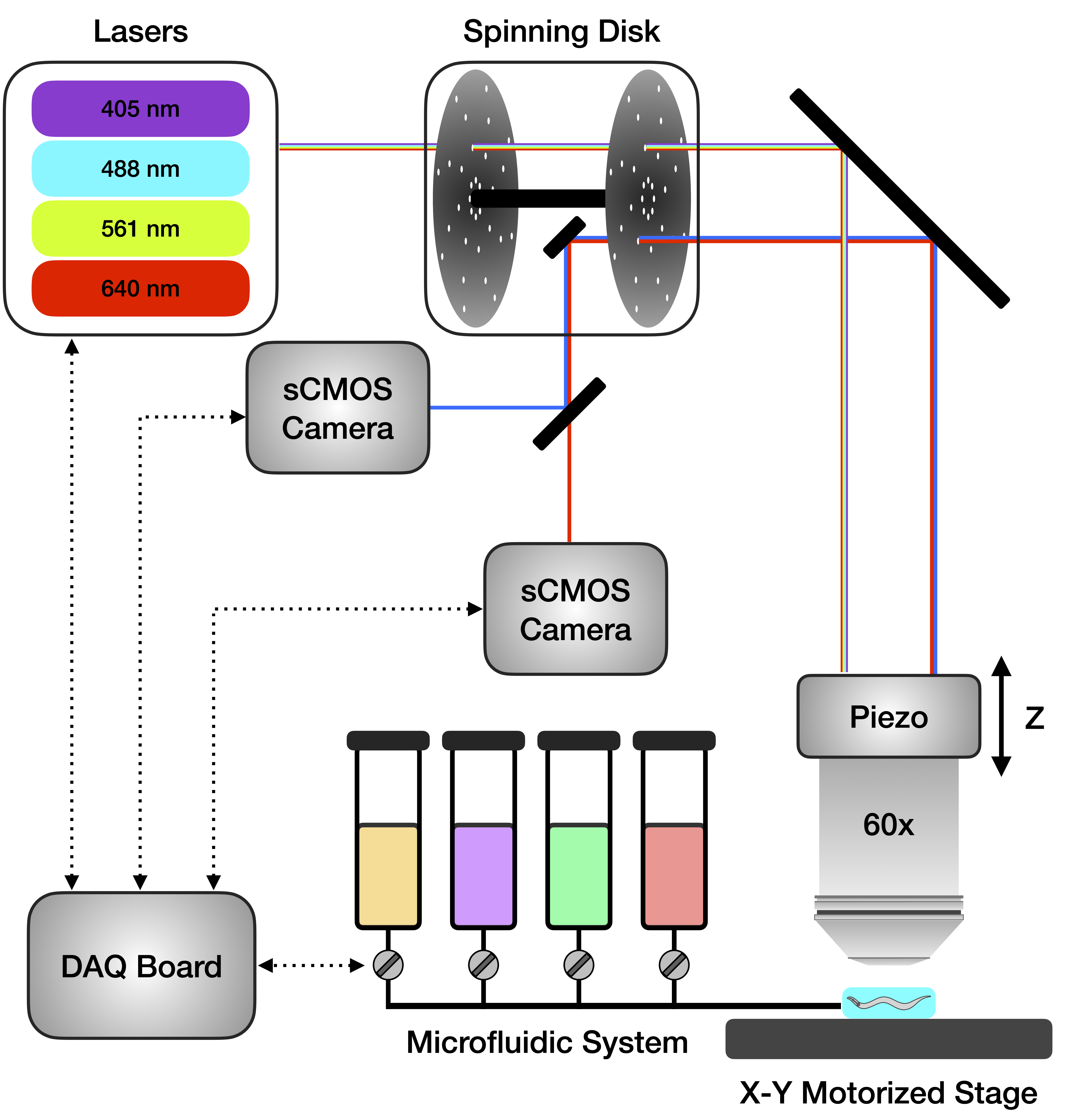
Currently, a member of Venkatachalam Lab, trying to understand the brain and the behavior of C. elegans, e.g. by building microscopes, designing experiments, and analyzing all sorts of data.
Making coffee for the lab members
 ,
while codes are running or models are training
,
while codes are running or models are training
 .
.

Mahdi Torkashvand
Mahdi has a PhD in physics and is currently a data scientist at Quiver Biosciences, where he is contributing to drug development efforts aimed at treating epilepsy and other neurological disorders. Mahdi was not involved in making this website, but he built the spinning-disk confocal microscope that was used to record all the datasets in this project. We are forever grateful and love him for that!